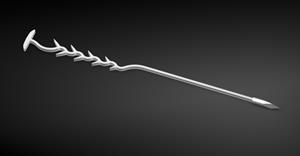
Spirair Receives FDA Clearance for TurbAlign, a New Bioabsorbable Implant That Helps Keep Nasal Passages Open After Sinus Surgery
SOUTH SAN FRANCISCO, Calif., July 21, 2025 (GLOBE NEWSWIRE) -- Spirair, Inc., an early-stage medical device company developing minimally invasive solutions to help otolaryngologists (ENTs) improve patient care, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its TurbAlign™ Implant, a novel, bioabsorbable device indicated to aid healing from sinus surgery by separating the middle turbinate from the lateral nasal wall during the critical healing period.
“After routine sinus surgery, scarring inside the nose or shifting of important tissues like the middle turbinates—structures that help regulate airflow—can affect healing and overall success,” said Jayakar Nayak, MD, PhD, Associate Professor of Otolaryngology at Stanford Medicine. “TurbAlign is an innovative, sturdy yet resorbable implant that helps secure these tissues to prevent unwanted adhesions, while preserving open sinus passages.”
TurbAlign is inserted by the physician with a simple, one-pass, knot-free technique. The implant provides secure middle turbinate medialization then resorbs without the need for removal.
“Our expanding portfolio highlights our commitment to ENT solutions that enhance patient care,” said Ben Bishop, CEO of Spirair. “TurbAlign provides a novel approach to middle turbinate medialization, while SeptAlign™ targets septal deviation. Together, they provide complementary advancements in nasal and sinus surgery.”
Commercial availability for TurbAlign is anticipated in the U.S. later this year.
About TurbAlign™
TurbAlign™ is an innovative, bioabsorbable implant used to separate the middle turbinates from the lateral nasal wall, helping maintain an open nasal cavity after sinus surgery. Inserted by the physician with a simple, one-pass, knot-free technique, it provides secure middle turbinate medialization then resorbs without the need for removal. The TurbAlign implant received FDA Clearance on July 11, 2025 and will be commercially available in the United States later in 2025. For full indications information, visit Spirair.com/TurbAlign.
About SeptAlign™
SeptAlign is a proprietary bioabsorbable implant developed to correct cartilaginous nasal septal deviations. The implant is inserted in the cartilage near the front of the nose and holds the straightened septum under tension, dissolving within six months. Its innovative design allows ENT specialists to realign and support the nasal septum, including challenging anterior caudal deviations, while preserving cartilage. SeptAlign can be used in any site of service as part of standard septoplasty techniques, including the office, the operating room (OR), and the ambulatory surgery center (ASC). The SeptAlign System received FDA clearance in 2024 and is commercially available in the United States. Visit Septalign.com for more information.
About Spirair®
Spirair, Inc. is an early-stage company pioneering minimally invasive solutions that help ENTs advance care for their patients by providing strong outcomes and a better patient experience. Spirair is committed to developing new therapies that can easily integrate in any ENT’s treatment algorithms so they can help more of their patients breathe better. The company was founded in 2020 by James Kintzing, PhD and Brandon McCutcheon, MD out of the renowned Stanford Biodesign Innovation Fellowship and Fogarty Innovation programs. Visit spirair.com for more information.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0ff00ed1-38f2-4cd9-883b-03ead2c3d3dd

Contacts Company contact: James Kintzing james@spirair.com PR contact: Vasey Hargreaves public_relations@spirair.com
TurbAlign implant
TurbAlign™ is an innovative, bioabsorbable implant used to separate the middle turbinates from the lateral nasal wall, helping maintain an open nasal cavity after sinus surgery. Inserted by the physician with a simple, one-pass, knot-free technique, it provides secure middle turbinate medialization then resorbs without the need for removal. The TurbAlign implant received FDA Clearance on July 11, 2025 and will be commercially available in the United States later in 2025. For full indications information, visit Spirair.com/TurbAlign.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.